Serological diagnosis of autoimmune pulmonary alveolar proteinosis
Serum anti-GM-CSF autoantibody concentration – the ELISA method
To confirm the diagnosis of autoimmune pulmonary alveolar proteinosis, which accounts for 92% of pulmonary alveolar proteinosis, it is necessary to measure anti-GM-CSF autoantibodies in the serum. Previous research has shown that there are three types of antibodies: IgG, IgM, and IgA, with the IgG type considered important in the pathogenesis, while the IgM type is a bystander at the onset of the disease (Nei et al., Am. J. Physiol. Lung, 2012).
The role of IgA-type autoantibodies is not well understood. It is known that the neutralizing ability of anti-GM-CSF autoantibodies is extremely high and their binding affinity to GM-CSF is extremely strong (Uchida et al., Blood, 2004).
Commercially available anti-GM-CSF autoantibody measurement kits are illustrated in the diagram below.
A 96-well plate fixed with recombinant GM-CSF is coated with a special blocking agent to prevent non-specific IgG binding, and the subject's serum, diluted 210 times, is added to bind to the fixed GM-CSF. After thorough washing, diluted peroxidase-labeled anti-IgG antibody is added to each well, and IgG anti-GM-CSF autoantibodies bound to GM-CSF are detected by adding substrate.
For measurement, a standard human monoclonal anti-GM-CSF antibody of known concentration is simultaneously reacted, a standard curve is drawn, and the autoantibody concentration in the subject's serum is determined.
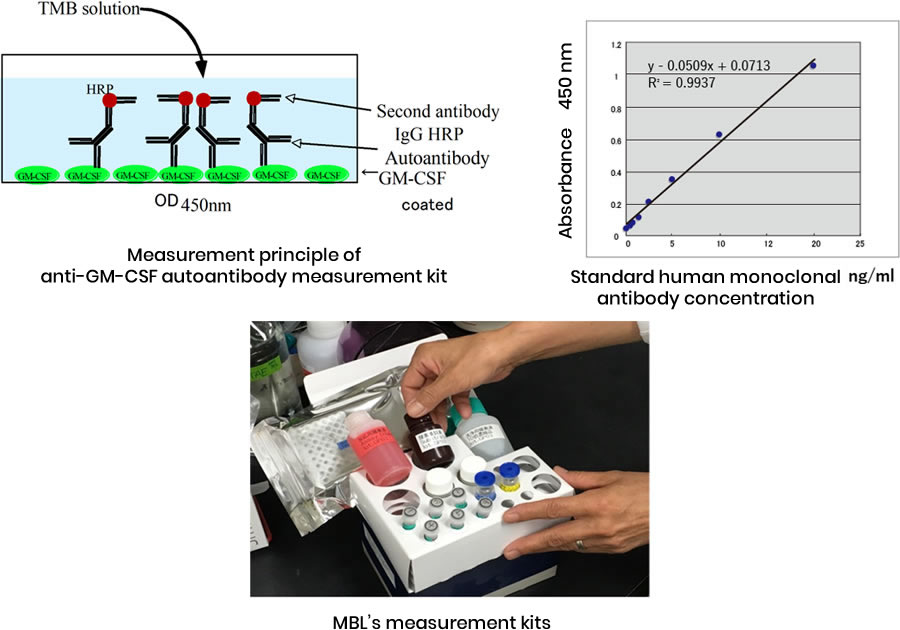
- This kit was released by the Medical and Biological Research Institute in 2020, and the testing company SRL purchased it and conducts measurements on contract.
- Sensitivity is 100% and specificity is 97%.
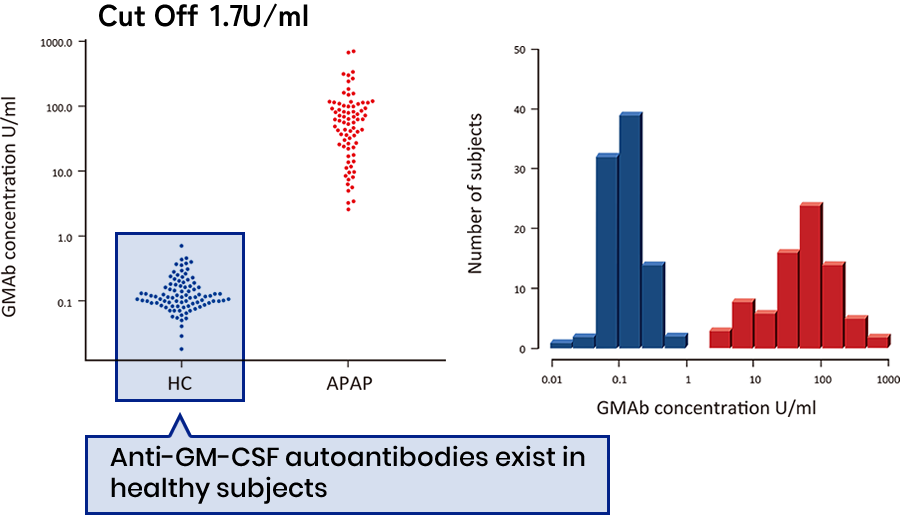
- Although very low, anti-GM-CSF autoantibodies are detected in the serum of healthy individuals at 0.15±0.11 U/ml (maximum 0.71, minimum 0.018 U/ml, N=90).
- However, in the serum of patients with autoimmune alveolar proteinosis, anti-GM-CSF autoantibodies are detected at 90.95±123.0 U/ml (maximum 718.7, minimum 2.59 U/ml, N=78).
- The cut-off value of serum from healthy subjects and patients with autoimmune alveolar proteinosis is 1.7 U/ ml, from logistic regression analysis.
Comparison of serum anti-GM-CSF autoantibody concentrations in lung diseases
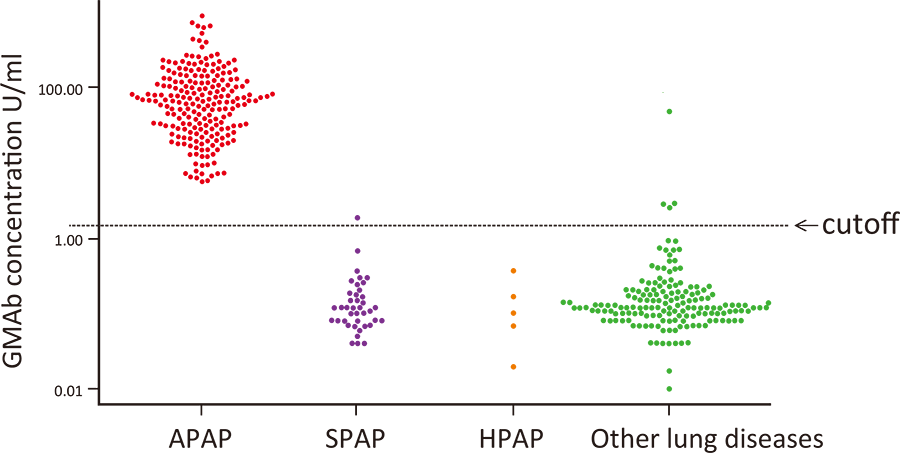
As shown in the graph above, we compare serum anti-GM-CSF autoantibody concentrations in 213 cases of autoimmune, 40 cases of secondary, five cases of hereditary, and 167 cases of lung diseases that require differentiation diagnosis from pulmonary alveolar proteinosis.
Logistic regression analysis using these data predicts the probability of disease onset for various antibody concentrations.
If a patient's serum anti-GM-CSF autoantibody concentration is 15.0 U/ml, the probability that the patient has autoimmune alveolar proteinosis is 90%, and if it is 21.0 U/ml or higher, it is 99%.
Of course, this probability may change if we increase the number of samples from other lung diseases compared. At the moment, this is just a probability based on these data.
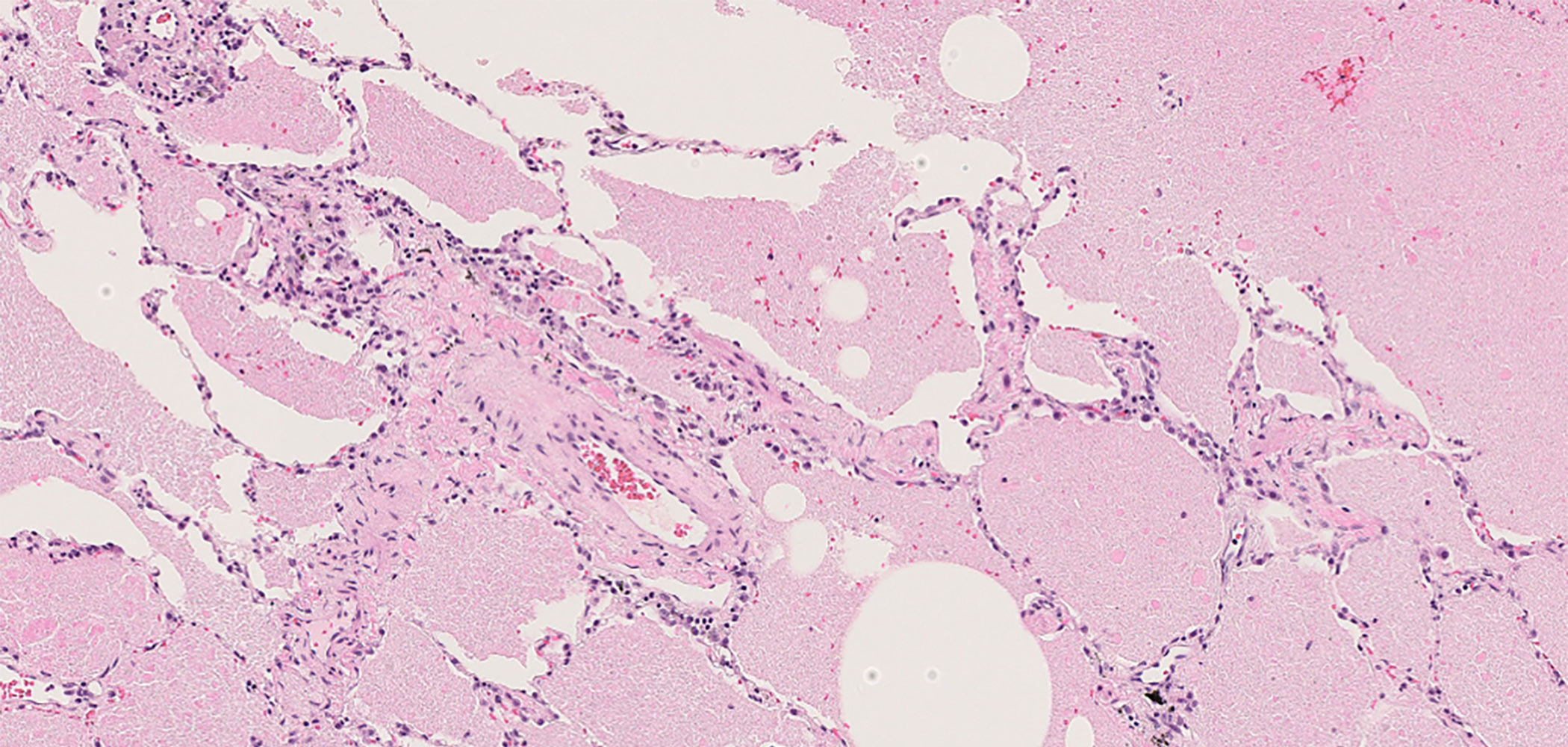
For clinical diagnosis, Ground-glass opacity (GGO) are observed on HRCT, serum anti-GM-CSF autoantibody concentration is measured, and if it is 1.7 U/ml or more, but less than 15.0 U/ml, the appearance of bronchoalveolar lavage fluid or lung biopsy may be considered. A diagnosis of autoimmune pulmonary alveolar proteinosis is made, based on these findings, and if the result is 21.0 U/ml or higher, although there is less than a 10% chance that this is not the case, it can be said that autoimmune pulmonary alveolar proteinosis is strongly suspected.