Simple serological diagnosis of autoimmune alveolar proteinosis
Nobel Pharma commercialized a kit that can easily determine whether anti-GM-CSF autoantibodies in serum are positive or negative using immunochromatographic assay.
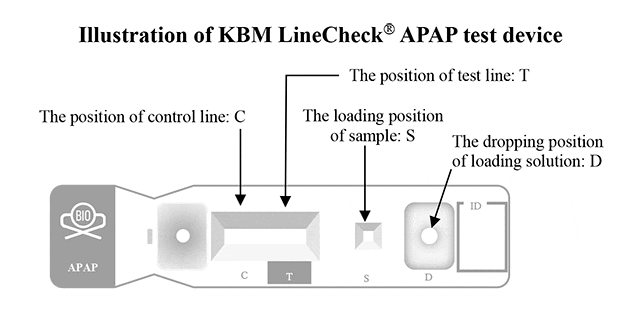
This product is designed based on an immunochromatographic assay that utilizes capillary action and antigen-antibody reactions. The test uses anti-human IgG antibody and horse anti-mouse IgG (H+L) immobilized on a nitrocellulose strip.
For more information about this kit, please see the following URL:
https://onlinelibrary.wiley.com/doi/10.1002/resp.70137
Rapid Serological Detection of Anti-GM-CSF Autoantibodies in Autoimmune Pulmonary and Alveolar Proteinosis Using a Novel Immunochromatographic Test - Narita et al. - Respirology - Wiley Online Library
The principles and demonstration of the kit are also available in the attached video abstract.
Video Abstract
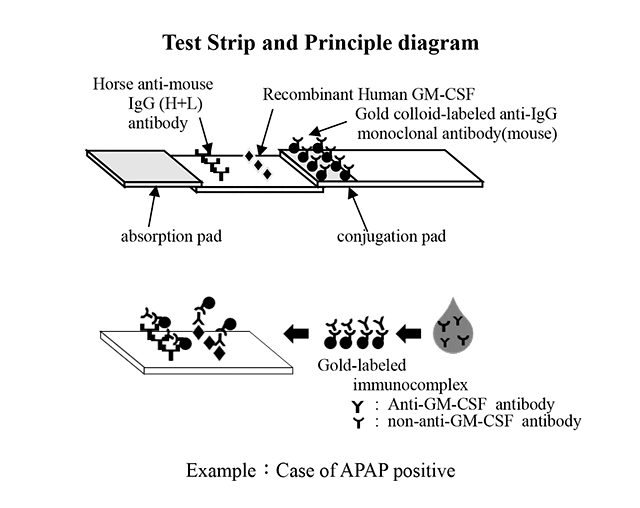
Anti-human IgG is coated in the position of the test line, and horse anti-mouse IgG (H+L) is applied in the position of the control line. The anti-GM-CSF antibodies in specimens that spot on the loading position of the test device react with gold colloid-labeled anti-IgG monoclonal antibodies. The gold-labeled immunocomplexes are then generated within the conjugation pad, and the complexes are moved by adding the reagent in the loading solution included in the kit.
The immunocomplexes then migrate toward the absorption pad on the membrane chromatographically, by capillary action, and react with the recombinant human GM- CSF in the test line position. If the specimen contains anti-human GM-CSF antibodies, a colored line consisting of the gold-labeled immune complexes will appear in the test line position.
If the specimen does not contain anti-GM-CSF antibodies, no colored line will appear in the test line, indicating a negative result. To serve as a procedural control, a colored line will always appear in the control line position, indicating that the proper volume of the specimen has been added and that the capillary phenomenon has occurred in the membrane.
Figure Source : Nobelpharma Co., Ltd. (Sargumarin product information / KBM line check APAP)
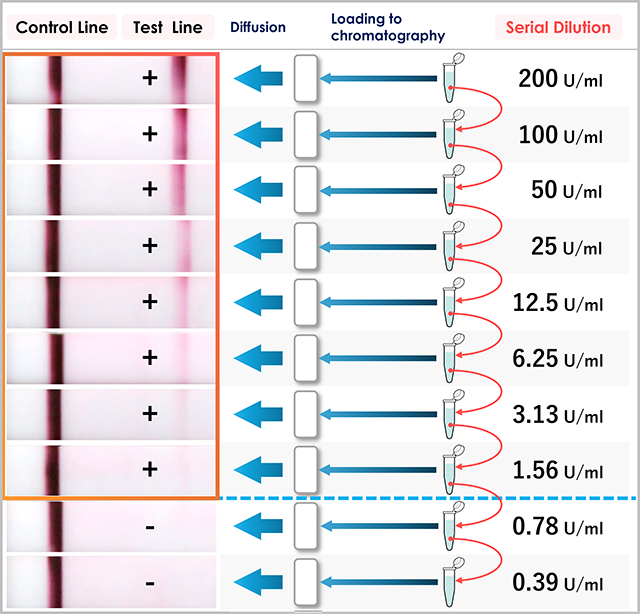
When we serially dilute the serum containing 200 U/ml of anti-GM-CSF autoantibodies with human serum, we find that the test line is visible up to 1.56 U/ml, and any further dilution will result in a negative test line (0.78 U/ml and 0.39 U/ml are below the lower measurement limit). In other words, if you can see the test line, the serum has a concentration of 1.56 U/ml or higher.
Note: This immunochromatographic assay was developed to detect anti-GM-CSF autoantibodies in serum and is not suitable for the detection of other samples such as bronchoalveolar lavage fluid, ascites fluid, or pleural fluid.