About whole lung lavage for pulmonary alveolar proteinosis
Whole lung lavage is one of the treatments for pulmonary alveolar proteinosis. Whole lung lavage is a procedure that removes abnormal accumulations of waste materials from the lungs under general anesthesia. Generally, the left and right lungs are separately lavaged.
A double-lumen tube is intubated into the patient's trachea, and the non-irrigated tube is connected to a ventilator to control breathing. Physiological saline is injected into the tube and drained after a certain period of time to flush waste products accumulated in the alveoli.
By repeating this operation 10 to 20 times, waste materials accumulated in one lung will be removed to some extent.
Whole lung lavage has been reported to be highly effective for the treatment of autoimmune pulmonary alveolar proteinosis, but the procedure is complicated. Since it is necessary to use a ventilator under general anesthesia, there is a risk of complications if the procedure is not performed by a team of doctors who are proficient in the procedure.
The efficacy of whole lung lavage
John F. Seymour et al. published “State of the Art: Management of Pulmonary Alveolar Proteinosis” (Am J Respir Crit Care Med , Vol 166. pp 215–235, 2002 DOI:
10.1164/rccm.2109105) in 2002, in which it was described that lung lavage has a significant impact on patient prognosis.
Regardless of the severity of alveolar proteinosis, the 5-year survival rate for the 146 patients who underwent lung lavage at some point during their course was 94 ± 2%. In the 85 patients without lavage, it was 85 ± 5%. However, recurrence may occur after lung lavage, in which case another treatment may be required.
In 84% of patients, symptoms, respiratory function, and chest image findings improved within 12 months after the first lavage. However, 66% of all patients required a second or subsequent lung lavage more than one year after diagnosis. In other words, although most patients responded to lung lavage and recovered, in two-thirds of cases, patients did not recover within a year. This means that over time, such patients have to undergo whole lung lavage again.
There were no differences in symptoms, duration of symptoms, smoking status, time from diagnosis to whole lung lavage, A- aDO2, serum LDH, and reported age between responder and non-responder. The patients who did not improve tended to be younger (median: 35 years vs. 39 years). The time for improvement with whole lung lavage was about 15 months. When we checked the treatment response to lung lavage by dividing the age group into 20 years old or younger, 21 to 39 years old, and 40 years old or older, the treatment effects tended to be more effective with increasing age: 58% (7 out of 12 cases), 84% (42 out of 50 cases), and 90% (43 out of 48 cases), respectively.
An international study on whole lung lavage for pulmonary alveolar proteinosis, published in 2016 (Campo et al., Orphanet J. Rare Dis., 11:115.DOI: 10.1186/s13023-016-0497-9), reported that whole lung lavage in adults had been carried out in 20 facilities in 14 countries and in 10 facilities in six countries. Reasons given for performing whole lung lavage were reduced lung function in 100% of cases, decreased resting PaO2 in 90% of cases, worsened chest image findings in 79% of cases, and progressive subjective symptoms (mainly dyspnea) in 42% of cases. On the other hand, in JRS guidelines, the standard indication for whole lung lavage is PaO2<70 Torr under room air while lying at rest, and the disease progressed. Contraindications for whole lung lavage include severe cardiovascular disease, heart failure, sepsis, severe lung infection, and terminal fibrosis. In addition, when performing whole lung lavage, pulmonary non-tuberculous mycobacterial disease or pulmonary aspergillosis infection should be treated and then carefully considered.
Whole lung lavage technique
Accurate insertion of the intubation tube requires an advanced technique. For the double-lumen tube for single-lung isolated ventilation, in general, the left-hand one is used regardless of whether washing the left or right side. This is due to the anatomical characteristics of the bronchus (the left main bronchus is more important than the right main bronchus). This is because the main bronchus is about three times longer and the tube can be fixed stably. Fixing this securely to the bronchus prevents leakage of lavage fluid into the ventilated lungs. Additionally, it is generally recommended that the cuff pressure of the tracheal tube is less than 30 cm H2O. To prevent leakage of lavage fluid into the ventilated lung, the irrigated lung is pressurized with 30–40 cm H2O to ensure that there is no air leakage from the ventilated lung. Artificial ventilation management during whole lung lavage basically uses pressure control ventilation +PEEP.
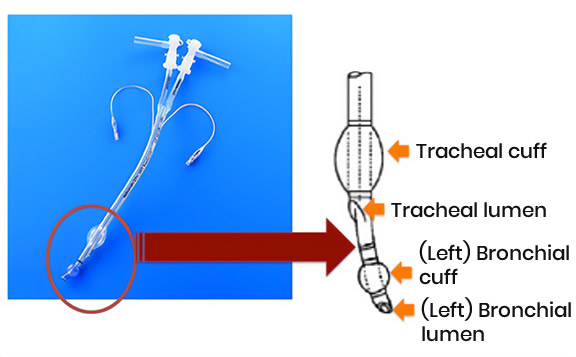 Figure : double-lumen tube for single-lung isolated ventilation
Figure : double-lumen tube for single-lung isolated ventilationBefore the first saline injection, a step called "degassing" is usually performed. In this step, the lavage side of the lung is ventilated with 100% oxygen to remove the air (oxygen + nitrogen) in the lungs. This is because the flow of washing fluid into the alveoli is inhibited by the remaining air, reducing the cleaning effect. After the lavage lung is ventilated with 100% oxygen for 15 minutes, the lavage tube is clamped for 10 to 15 minutes to create absorptive atelectasis. By carrying out this step, the lavage fluid will flow evenly into the lungs, maximizing the lavage effect. Rapid atelectasis formation may cause hypoxemia. Therefore, it is also important to monitor the patient's oxygen saturation and respiratory status during degassing.
Regarding the body position during washing, international studies have shown that 12 of 20 medical institutions adopted the supine position, six institutions adopted the lateral position at 30 to 45 degrees, and two institutions adopted the lateral position at 30 to 45 degrees. Seven institutions adopted the LUG position (a position in which the lower body is kept higher than the head while lying on the back). The supine position is appropriate for institutions with little experience.
 Connecting the cleaning solution (saline)
Connecting the cleaning solution (saline) to the cleaning tube and initiating the infusion.
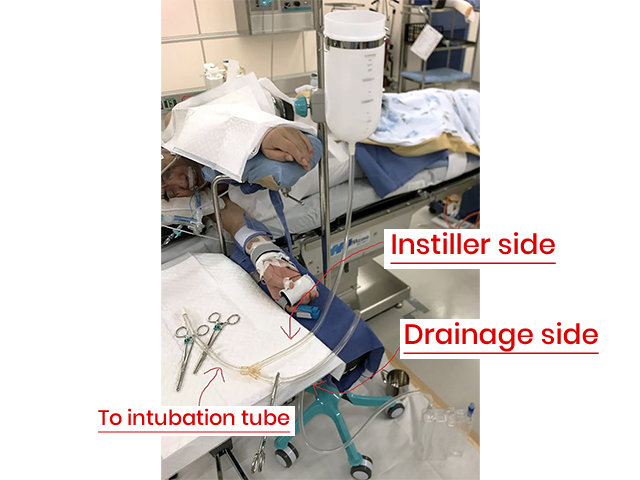
 Drainage tube height -
Drainage tube height - The drainage bottle is grounded to the floor.
All 20 facilities mentioned above used saline heated to 37 ℃. The amount of saline injected per session varied greatly depending on the facility, with an average of 800 ± 331 mL. Therefore, the total volume injected also varied widely, ranging from 5 to 40 L, with a mean total volume of 15.4 ± 6.8 L per lung.
Most (14/20) facilities used intraoperative chest percussion to increase cleaning efficiency. However, how and when this happened varied widely between facilities. Ten (50%) facilities used manual tapping, and four (20%) used vibrators.
Injection and drainage of saline are performed as follows:
- A lavage solution (normal saline) warmed to 37 ℃ is injected into the lavage side of the lung through a double-lumen intubation tube.
- The lavage fluid is allowed to stay in the lungs for a few minutes. At this time, vibrations are applied from outside the body to stir the washing solution.
- The washing solution is drained naturally from the height of the bed to the floor. At this time, not all the injected fluid is drained, but a certain amount of lavage fluid remains in the lungs.
- When most of the washing solution is drained, the next saline is added, and washing continues in the same way.
- The effectiveness of washing is usually judged by the change in turbidity of the draining fluid.
- The fluid drained during early washing is usually cloudy and contains a lot of precipitation. As washing proceeds, the turbidity of the drained fluid will become lighter and more transparent. Turbidity is often assessed to judge the timing of lavage termination, but there are no established criteria for termination. Essentially, the appearance becomes more transparent, there are fewer changes in the appearance by each cycle, and it ends after a certain number of cycles.
The duration of whole lung lavage for one lung varies from 2 to 6 hours. After the whole lung lavage is completed, the tube is replaced with a single-lumen tracheal tube and antibiotics are administered (in some facilities, antibiotics are administered after the first lavage fluid is drained).
 Bronchoalveolar lavage fluid after 20 lavages
Bronchoalveolar lavage fluid after 20 lavagesRecovery period
After washing, the patient will be kept on artificial respiration for 12 to 24 hours in the ICU and will be discharged after confirming that oxygenation has improved. International studies have reported that the most common complication of whole lung lavage is transient fever, followed by hypoxemia and wheezing. It has been reported that whole lung lavage is performed an average of 2.5 times per patient (counting left and right lavage as one time), and in approximately 2/3 of cases it has been performed more than once, with an average interval of 8.0 ± 6.5 months between lavages. In the report by Ichiwata et al., the average number of whole lung lavages performed was 2.60 per patient, and the average interval between whole lung lavages was 15.0 months.
Complications during whole lung lavage
One potential difficulty during whole lung lavage is hypoxemia due to leakage of infused lavage fluid into the ventilated lungs when the cuff pressure is low. Additionally, if lavage fluid leaks into the ventilated lungs, the artificial nasal filter becomes excessively humidified and becomes obstructed, impairing ventilation. Therefore, it is important to adjust the irrigation tube position properly and cuff pressure to minimize leakage. During lung lavage, it is important to record the amount of lavage fluid injected and drained, to know how much lavage fluid remains in the lungs.
In general, the following complications and troubles have been reported with whole lung lavage:
- 1. Hypoxemia
- This can occur during injection of lavage fluid or degassing (creating absorptive atelectasis after injecting 100% oxygen). It is necessary to take into consideration the increase or decrease in blood flow in the lavage lungs, which becomes shunt blood flow. Body positioning during lavage may reduce shunt blood flow and prevent severe hypoxemia.
- 2. Infectious disease
- Postoperative (ventilator-associated) pneumonia may occur. For this reason, antibiotics are given.
Although the frequency of the following complications is low, it is necessary to take them into consideration: - 3. Bleeding
- Bleeding in the lungs and airways may occur during irrigation. If the bleeding is mild, irrigation may continue; if the bleeding is severe, it should stop.
- 4. Pneumothorax
- Pneumothorax may occur during irrigation. Attention must be paid to changes in drainage volume or circuit pressure on the washing side and to changes in ventilation volume and airway pressure on the ventilation side. It is important to perform a chest X-ray after cleaning to check for pneumothorax.
- 5. Catheter-related complications
- When using extracorporeal membrane oxygenation (ECMO) in combination, vascular damage, embolism, and thrombosis may occur when inserting a catheter.
- 6. Pulmonary embolism
- A pulmonary embolism may occur during lavage. This can be prevented by adjusting the flow rate and injection amount of the cleaning solution.
- 7. Airway spasm
- Airway spasm may occur during irrigation. In this case, bronchodilators should be administered. In addition, if physiological saline is injected without being heated to 37 ℃, the airway temperature may drop rapidly and airway spasm may occur.
These complications can be prevented if the doctors and nurses who insert the irrigation tube and inject and drain the irrigation solution have appropriate skills and knowledge. It is also important to perform appropriate patient evaluation and risk management before the lavage.
Regarding the use of ECMO*
* ECMO=extracorporeal membrane oxygenation
In the past, ECMO use was often avoided in patients with advanced respiratory failure due to the risks. However, in recent years, it has become possible to perform whole lung lavage using ECMO (heart-lung machine).
ECMO is used to support patients with respiratory failure by removing blood from the body, providing oxygen, and removing carbon dioxide. Using ECMO during whole lung lavage allows lavage to be performed while increasing patient safety.
Since ECMO is a treatment for serious conditions, there is a wide range of complications.
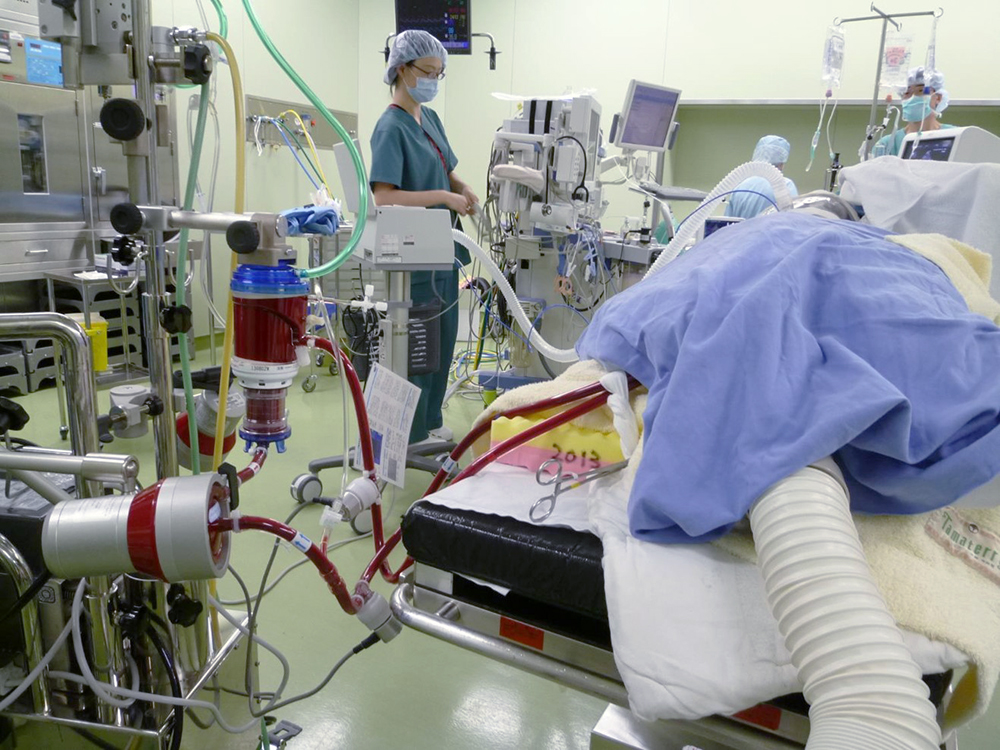 A patient undergoing ECMO.
A patient undergoing ECMO. The patient's blood is being perfused through an ECMO circuit from vein to vein.
In recent years, the frequency of using ECMO has increased due to factors, such as COVID-19 infection, so management methods have been established, and the risk of complications has decreased with advances in equipment.
However, typical complications include the following:
- 1. Bleeding
- There is a risk of bleeding due to blood vessel damage when inserting the catheter. To minimize the risk of circulation blockage, anticoagulants are used during ECMO, which makes bleeding more likely than usual.
- 2. Thrombosis
- Because ECMO slows blood circulation and because blood is circulated outside the body, thrombosis may occur. Therefore, anticoagulants may be used to prevent thrombosis.
- 3. Infectious disease
- To perform ECMO, an intravenous catheter is inserted. Therefore, there is a risk of infection. Precautions include keeping the intravenous catheter clean and using appropriate antibiotics.